Oxidation Number of Carbon
Emerging single atom catalysts SACs especially carbon-based SACs are appealing materials in environmental catalysis because of their ultrahigh performances environmental friendliness structuralchemical robustness and the maximum utilization of active metal sites. Since the electrons between two carbon atoms are evenly spread the R group does not change the oxidation number of the carbon atom its attached to.
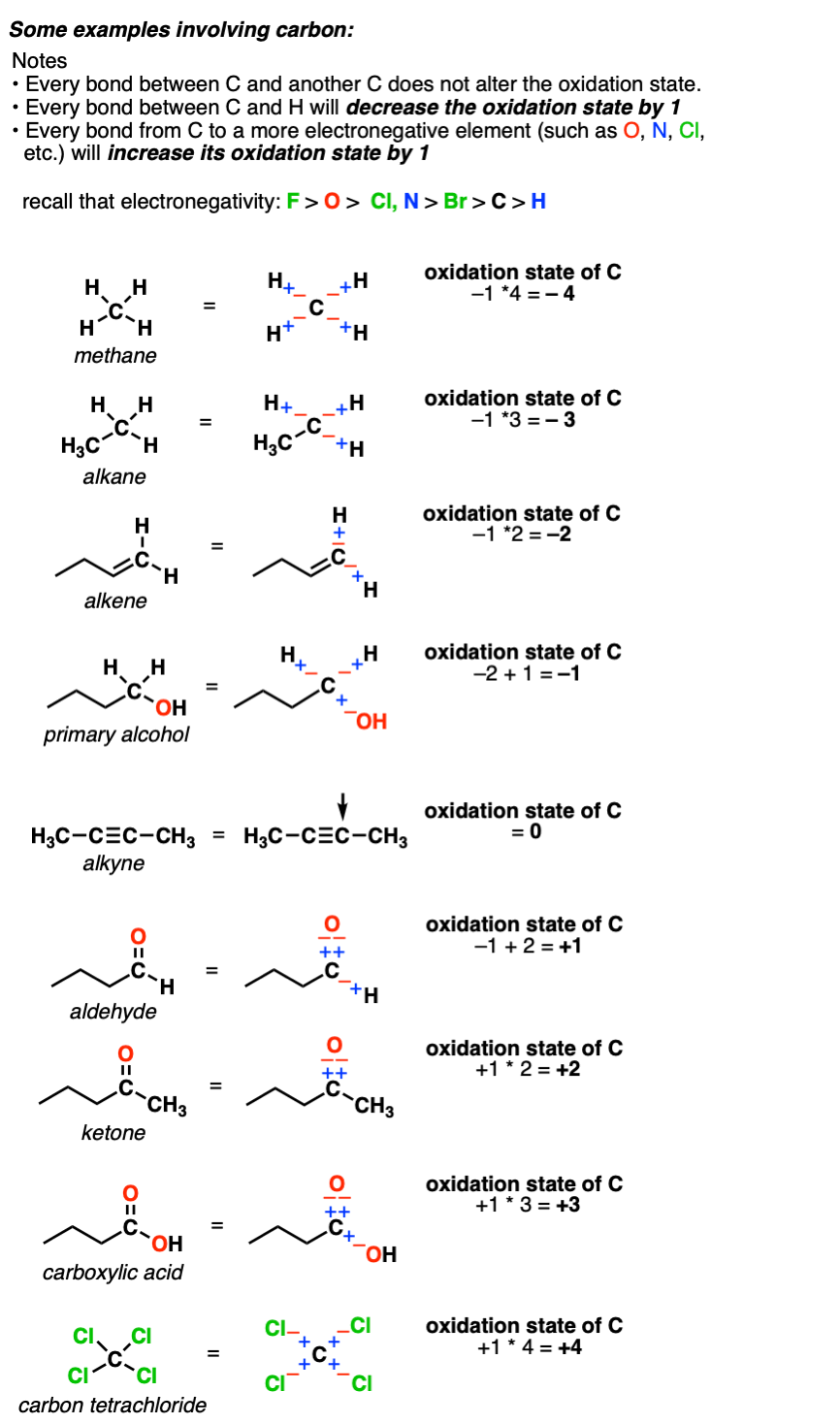
Calculating The Oxidation State Of A Carbon Master Organic Chemistry
Advanced oxidation processes AOP can be defined as a set of emerging chemical procedures which can remove soluble organic effluents from water and soil.

. Thus the valency of nitrogen is 3 whereas it can have oxidation numbers from -3 to 5. Carbon has atomic mass 12 and atomic number 6. Here we demonstrate the synthesis of value-added aldehydes and carboxylic acids with clean hydrogen H 2 production in water using a photoelectrochemical cell based solely on polymeric carbon nitride CN as the photoanode.
Carbon in organic molecules can have any oxidation number between -4 and 4. The number of acetyl-CoA produced depends upon the carbon length of the fatty acid being oxidized. An atom that accepts an electron to achieve a more stable configuration is assigned an oxidation number of -1.
C 2 H 6. The general form is X O 2 CO 2 H 2 O. Valency is different from the oxidation number and it has NO SIGN.
The term covers a large and diverse body of processes. Photo-oxidation by ultra-violet UV light alone or with a catalyst. These reactions involve a fuel usually organic and oxygen as reactants and result in water and carbon dioxide and occasionally another organic product such as nitrogen.
Development in Wastewater Treatment Research and Processes 2022. The oxidation number is the hypothetical charge of an atom in a molecule or ion and it is a measure of its apparent capacity to gain or lose electrons within that species. ATP from fatty acid spiral 7 turns and 5 per turn 35 ATP.
CHEMICAL BONDING APPLICATION OF CHEMICAL REACTIONS and ORGANIC CHEMISTRY. Rules for assigning oxidation numbers. On the other hand MPs having low ozone.
See the graphic on the left bottom. Remember that the number of turns is found by subtracting one from the number of acetyl CoA produced. In chemistry the oxidation state or oxidation number is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic.
It works by creating powerful fast-acting oxidizers called hydroxyls which are a combination of hydrogen and oxygen atoms found in the earths atmosphere. With Place Markers Off your results will look something like this. Carbon is a very important chemical element with a chemical symbol of CAll known life on Earth needs it.
One sulphur atom in Na 2 S 2 O 3 has an oxidation number of 6 whereas the other sulphur atoms have an oxidation number of -2. 27 Alcohols and carboxylic acids e oxidation of primary alcohols to aldehydescarboxylic acids and secondary alcohols to ketones. Atomically dispersed iron hydroxide deposited on silica-supported platinum nanoparticles enables complete and selective carbon monoxide removal through preferential oxidation in hydrogen in the.
5 3 42 18 20 This is the default layout Research Randomizer uses. Photo-chemical oxidation with UV and a chemical oxidizer usually a persulphate. Amines and carbon-carbon double bonds.
The oxidation number of a free element is always 0. It is a nonmetal meaning that it is not a metal. Oxidation-reduction reaction also called redox reaction any chemical reaction in which the oxidation number of a participating chemical species changes.
Example with Palmitic Acid 16 carbons 8 acetyl groups. As a result the average sulphur oxidation state number in Na 2 S 2 O 3 is 2. Advanced oxidation or AOP is a newer method in pool oxidation and has generated a lot of excitement recently within the pool industry.
ENERGY RATE AND CHEMISTRY OF CARBON COMPOUNDS. A number assigned to an atom describing its degree of oxidation meaning how many electrons it. Isotope labeling measurements and DFT calculations reveal a preferential adsorption of benzyl alcohol and molecular.
Number of turns of fatty acid spiral 8-1 7 turns. 2 17 23 42 50 Set 2. Oxidation State Number.
You can find examples of usage on the Divide the redox reaction into two half-reactions page. This process involves a variety of enzymes with the four main enzymes involved in fatty acid β-oxidation being in order acyl-CoA dehydrogenase enoyl-CoA hydratase hydroxyacyl-CoA dehydrogenase and ketoacyl-CoA thiolase Figure 3 11. The metal centres carbon matrixes an.
In HCHO the oxidation number of carbon is zero. When iron is alloyed with carbon hard steel. Keeping the atomic orbitals when assigning oxidation numbers in mind helps in recognizing that transition metals pose a special case but not an exception to this convenient method.
The UV oxidation method offers the most reliable low maintenance method of determining TOC in ultra-pure waters. C 2 H 4 CH 3 Cl. C 2 H 2 C 6 H 6 CH 2 OH 2.
Integer oxidation states of carbon Oxidation state Example compound 4. Many oxidation-reduction reactions are as common and familiar as fire the rusting and dissolution of metals the browning of fruit and respiration and. The donation of an electron is then 1.
Advanced Oxidation Process AOP systems. 25 CRUDE OIL FUELS AND ORGANIC CHEMISTRY. Thermo-chemical oxidation with heat and a chemical oxidizer usually a persulphate.
HCHO CH 2 Cl 2 1. Place Markers let you know where in the sequence a particular random number falls by marking it with a small number immediately to the left.
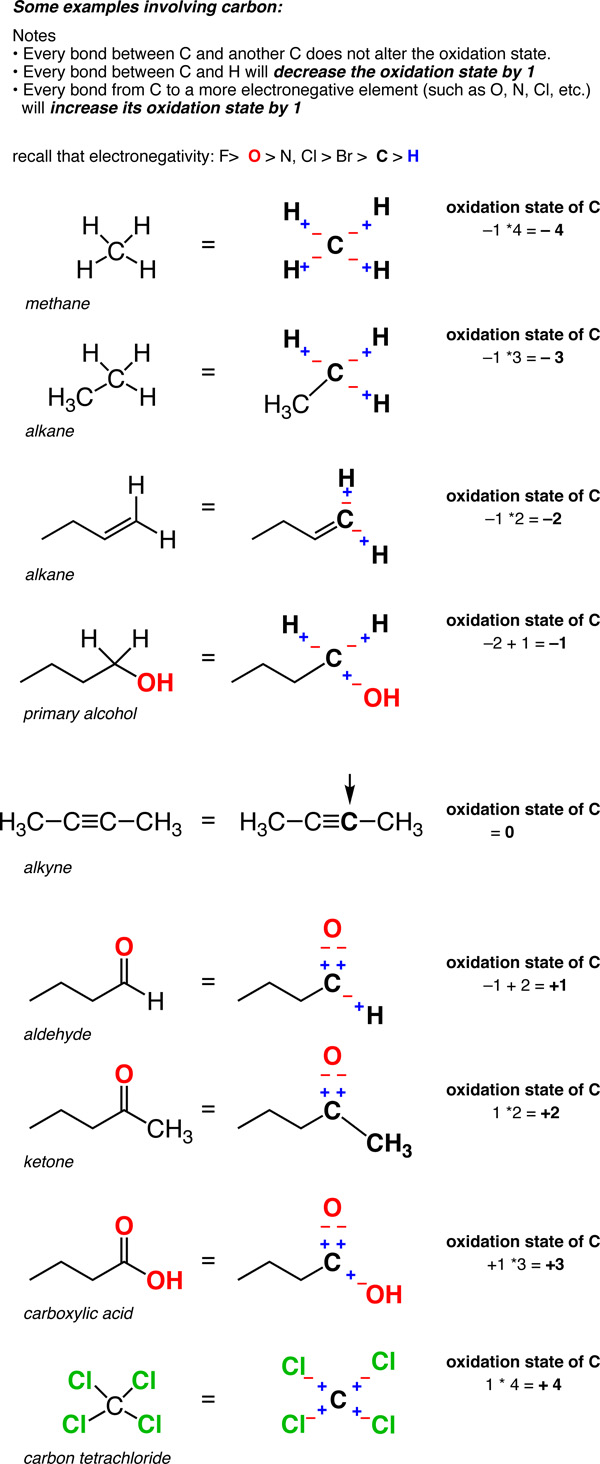
19 1 Definition Of Oxidation State For Carbon Organic Chemistry Ii

Oxidation Number How To Find Oxidation State
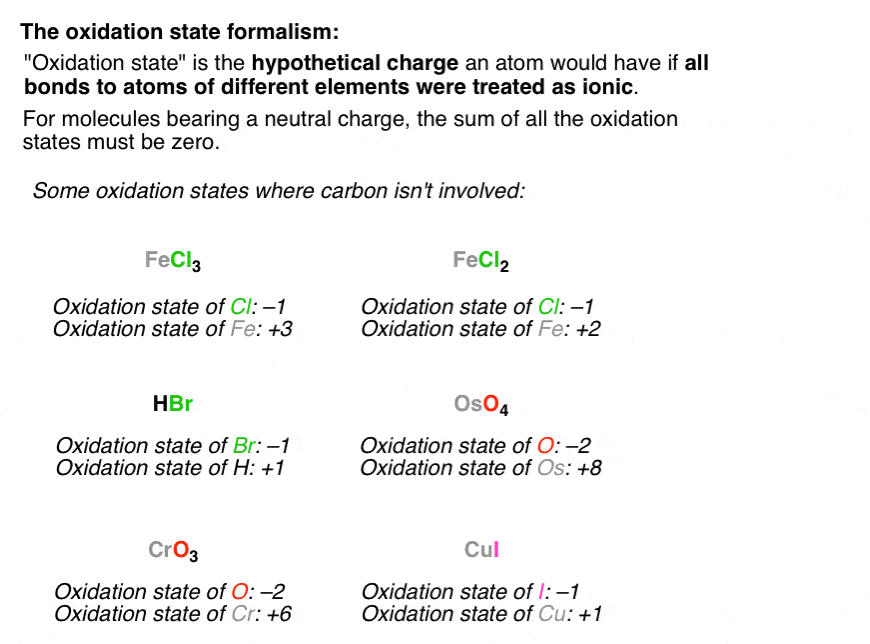
Calculating The Oxidation State Of A Carbon Master Organic Chemistry

Oxidation Number State Definition Rules How To Find And Examples
Comments
Post a Comment